Standard Electrode Potential
Standard Electrode Potentials In an electrochemical cell, an electric potential is created between two dissimilar metals. This potential is a measure of the energy per unit charge which is available from the oxidation/reduction reactions to drive the reaction.
redox reactions electrolysis The difference between electrochemical and electrolysis.
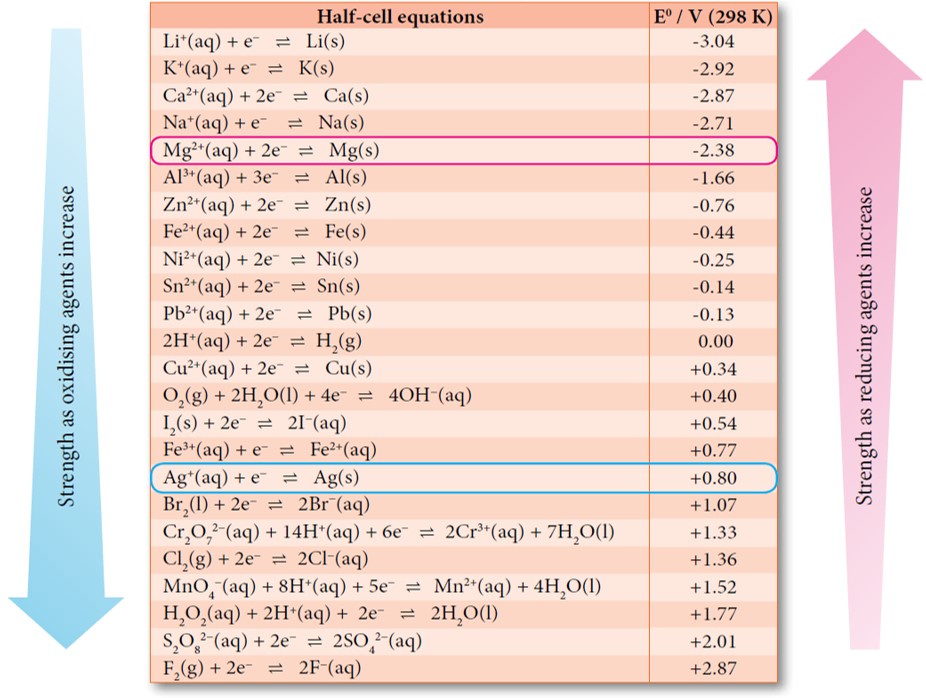
Table of standard electrode potentials Legend: ( s) - solid; ( l) - liquid; ( g) - gas; ( aq) - aqueous (default for all charged species); ( Hg) - amalgam; bold - water electrolysis equations. See also Galvanic series lists electrode potentials in saltwater Standard apparent reduction potentials in biochemistry at pH 7

Electrode Potential and Standard Electrode Potential EMF Embibe
In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be characterized. [1] By convention, the reference electrode is the standard hydrogen electrode (SHE). It is defined to have a potential of zero volts.

Appendix 4 Standard Reduction Potentials First Year General Chemistry Images
Essential Laboratory Skills Guide Weighing the right way These electrode potentials are given in volts relative to the standard hydrogen electrode. The values below are standard electrode potentials taken at 298 K, 1 bar pressure and in aqueous solution, of concentration 1 molar. [1] [2] [3] [4] References

Standard Electrode Potential Table JEE Main Electrochemistry Part4 Galvanized Cell
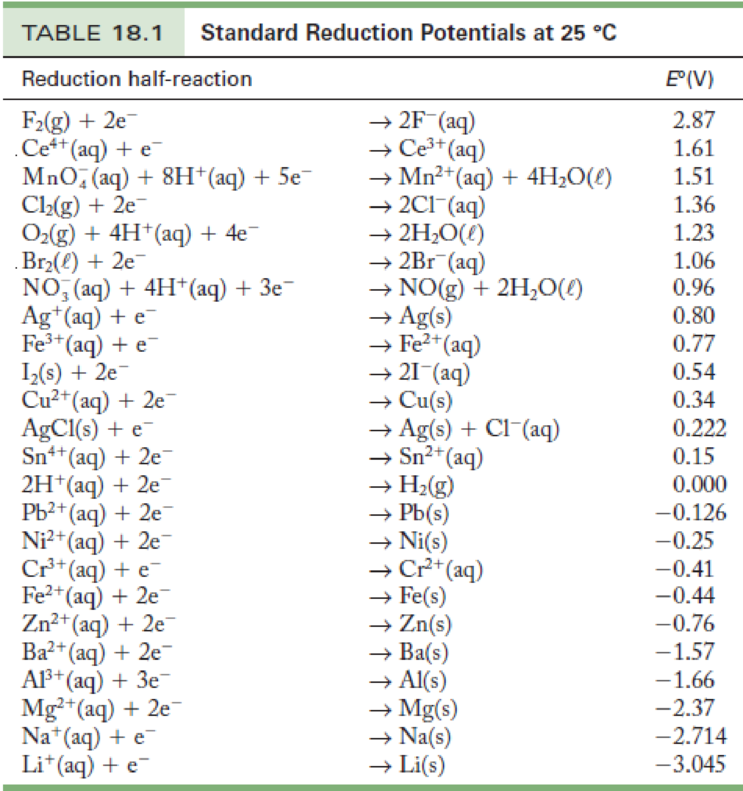
Standard Electrode Potentials in Aqueous Solution at 25°C Cathode (Reduction) Half-Reaction: Standard Potential E.
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K
Introduction; 18.1 Periodicity; 18.2 Occurrence and Preparation of the Representative Metals; 18.3 Structure and General Properties of the Metalloids; 18.4 Structure and General Properties of the Nonmetals; 18.5 Occurrence, Preparation, and Compounds of Hydrogen; 18.6 Occurrence, Preparation, and Properties of Carbonates; 18.7 Occurrence, Preparation, and Properties of Nitrogen

Solved Standard Electrode Potentials in Aqueous Solution at
Physical & Theoretical Chemistry Thermodynamics and Chemical Equilibrium (Ellgen) 17: Electrochemistry
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Presentation Free Online
The standard hydrogen electrode is a half-cell used as a reference electrode and consists of:. Hydrogen gas in equilibrium with H + ions of concentration 1.00 mol dm-3 (at 100 kPa); 2H + (aq) + 2e-⇌ H 2 (g). An inert platinum electrode that is in contact with the hydrogen gas and H + ions; When the standard hydrogen electrode is connected to another half-cell, the standard electrode.

Standard Electrode Potential Table JEE Main Electrochemistry Part4 Galvanized Cell
Figure \(\PageIndex{2}\): A cell permitting experimental measurement of the standard electrode potential for the half-reaction. Table \(\PageIndex{1}\) provides a listing of standard electrode potentials for a selection of half-reactions in numerical order, and a more extensive alphabetical listing is given in Appendix L.

PPT Electrochemistry PowerPoint Presentation, free download ID5368514
Tables of Standard Electrode Potentials. Journal of The Electrochemical Society , Volume 125 , Number 6 Citation G. Milazzo et al 1978 J. Electrochem. Soc. 125 261C DOI 10.1149/1.2131790.

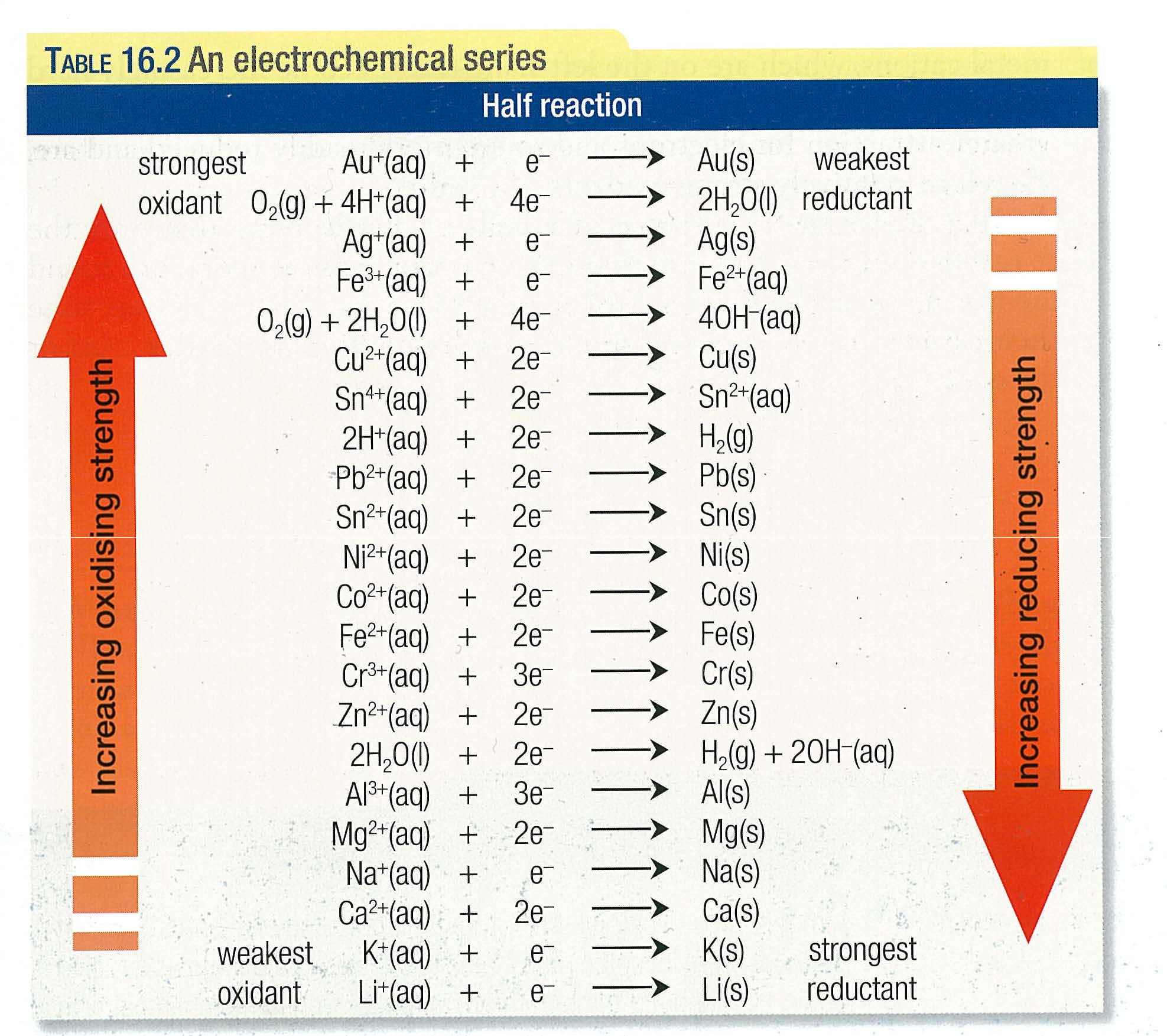
Electrochemical Series Electrochemistry, Chemistry, Chemistry notes
The Young's modulus, E, and Shear modulus, G data of various boron nanosheets are summarized in Table 6 44,45,46. The Young's modulus (N/m) indicates a material's ability to withstand.

Standard Electrode Potential Table ArjunldWilkinson
Electrode potential Measuring potential Electrical double layer Table of standard reduction potentials Download chapter PDF Electroneutrality One of the basic phenomena in nature is the preservation of electroneutrality , the tendency to discourage and oppose any processes that lead to an excess of positive or negative charge.

Standard Electrode Potential Table JEE Main Electrochemistry Part4 Galvanized Cell
It is well-known which the electrode materials play an important role in the manufacture of superior-efficiency electrochemical sensing platforms for detecting. potential (potentiometric), current. sensors. The data in Table 2, Table 3, Table 4, Table 5 confirm the efficiency of sensing systems based on TMD nanostructures.

Corrosion Science Demonstration
This page titled Standard Electrode (Half-Cell) Potentials is shared under a CC BY 4.0 license and was authored, remixed, and/or curated by OpenStax via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.

A Level Chemistry Electrodes & Electrochemical Cells
The following table provides E o for selected reduction reactions. Values are from the following sources: Bard,. New York, 1985; Milazzo, G.; Caroli, S.; Sharma, V. K. Tables of Standard Electrode Potentials, Wiley: London, 1978; Swift, E. H. Standard Reduction Potential E° (volts) Li + (aq) + e-\(\rightleftharpoons\) Li(s)-3.040: Rb.

Unit 7 Nuclear and Chemistry lessons, Teaching chemistry, Chemistry basics
The standard electrode potential, \(E^{\circ}\), for a half-reaction is the potential when all species are present at unit activity or, for gases, unit fugacity.. The appendix in Chapter 35.8 provides a table of standard state reduction potentials for a wide variety of half-reactions at 298 K.